- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
Crystallography in CanadaStanley C. Nyburg Stanley C. Nyburg, 66 Home Park Road, London, SW19 7HN, UK
I was born in London UK in 1924. My father, a clerk with J. Lyons & Co. the caterers, was an amputee, having lost his left arm at Ypres in World War 1. I gained admission to grammar school in 1936. In 1937 my father died and our family was left in straitened circumstances. In 1939 at the outbreak of World War 2 my school was evacuated to Weymouth on the south coast. Whilst there in May, we experienced the dreadful evacuation of troops (mainly French) from Dunkirk whom we helped to get fed and accommodated. In 1939 I took my matriculation examinations. We were examined on The Merchant of Venice during a heavy air-raid and almost certainly illegally were not sent to an air-raid shelter. We enjoyed the bombardment greatly. Oil tanks in the harbour were struck and the sky was so darkened we had to finish with all the lights on. I passed all the exams. By 1940 bombing was so severe we all had to return briefly to London.
From here we were re-evacuated to Winchester. Here I studied for advanced (university entrance) exams. To sit these we had to go to London University Examination Halls in July, 1942. I passed in Pure Mathematics, Applied Mathematics, Physics, Chemistry and Engineering Drawing. On this basis I was granted a university scholarship to read for a B.Sc. in engineering. However this was far from my first choice so that although my exam results qualified me for entrance elsewhere, lack of funds prevented it. Because of my father's connection J. Lyons & Co. asked me to work in their labs for them. I also enrolled at a part-time university college (Birkbeck, then in Fetter Lane) to read for an honours degree in chemistry with physics subsidiary.
I was 18 the following December and liable for call up to the armed forces. It was the government's Joint Recruiting Board which ruled on such matters and considering the dire peril of the war their attitude was liberal. Full time undergraduates once 18 would have to complete their studies in two years, part-time students in three. I was allowed to continue studying at Birkbeck in the evenings being at Lyons jam factory, later, labs in Hammersmith Road during the day. I found the work disagreeable and no help to my degree work. I asked the Recruiting Board to change my job and to help more in the war effort. My new job was with a Government Research Department (D.S.I.R). The Road Research Laboratory in Harmondsworth was quite a long way to travel and close to Heathrow Airport (only just started). Air raids over London were frequent and perilous and classes at Birkbeck were moved from evenings to weekends. After one intense air raid we had to sweep our lecture seats clean, and the lecturer in Physics having filled the blackboard slid it to one side. Thus was revealed a gaping hole (much of the external wall had been blown away) and there in all its glory was St Pauls Cathedral.
I passed the General B.Sc exam in physics, but decided to try full time studying. Middlesex Education Authority had no grant money but agreed to lend me enough, interest free, for one year's study. The Joint Recruiting Board generously conceded to allow me one year's extension. I accordingly, in October 1944, applied to and was accepted by King's College, London to read Hons B.Sc Chemistry. Although easier on my well being, studying was not easy because much of what was given in lectures I had already done and material that I needed had been given in earlier years.
Prof G M Bennett was Head of Department and lectured on Physical Methods. He dealt briefly with X-ray crystallography, already a powerful structural tool. He warned us not to get too heavily involved, a subject 'clearly beyond most of you'. Final exams were held at the same time as the war ended so I had no need to worry about call up to the armed services. After finals I wished to read for a Ph.D. but there were no graduate positions available. Prof Bennett came to my rescue. He found me an opening in August as crystallographic trainee at the British Rubber Producers' Research Association in Welwyn Garden City. (I did mention his earlier remarks about crystallography being 'difficult' but he laughed it off.) This opening was especially lucky for me. My salary was to exceed that of a graduate student's grant and I could read for a Ph.D. if supervised by someone qualified. This was because BRPRA was a recognised Institution of London University.
I here met George Jeffrey ('Jeff') who was to be my supervisor and also Andrew Booth (q.v.). I started X-Ray crystallography with primitive high-tension equipment on which I nearly killed myself. In November E. G. Cox (later Sir) was appointed to a Chair in Chemistry at Leeds University and asked Jeff to join him as Lecturer. Accordingly I went in November 1945 to live in Leeds and read for a Ph.D there. (My brief sojourn at BRPRA allowed me to be a humble co-author on my first paper, not published until 1948) (1)
Under Prof Cox's leadership there grew up a very effective X-ray crystallographic group. It was one of the first to semi-automate the summation of Fourier series. Hollerith cards were punched to correspond with Beevers-Lipson strips and these were summed on a card-reading machine used for accounting at the local electricity company (2). After a considerable struggle I managed, by photographic methods, to solve the crystal structure of an organic compound. The results were submitted for a Leeds Ph.D granted in 1949 (3).
In 1949 I was married and moved to St. Albans. I obtained an Assistant Lectureship at the new University College of N. Staffordshire at Keele to where we moved in 1952. Here I solved the natural rubber structure in 1954(4). I had started this work before at the BRPRA where I stretched a wide sheet of natural rubber on a frame and put it in a domestic freezer. Released from the frame the frozen rubber retained its shape. With suitably cold scissors a small fragment of frozen rubber was cut out. A skilful glass blower made a Dewar container, the inner chamber being connected to the outside by a glass spiral tube. A hyperdermic was affixed to the outside and when filled with liquid air the Dewar provided a continuous fine steam of very cold air. The rubber sample was mounted in this stream in a collimated beam of X-rays. Excellent diagrams were obtained at 30 deg rotational intervals and all intensities estimated by eye. The Bragg intensities can be well explained if the crystal structure is taken to be disordered. The isoprene units are planar as required but can occupy one of two possible positions. This structure has been widely accepted to be true.
From the 1954 published structure of rubber.
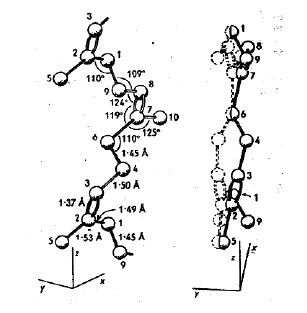
The correct molecular connectivity of aspidospermine as determined by crystallographic methods in the late 1950's.
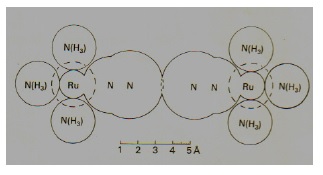
In 1958 G A Jeffrey had moved to the University of Pittsburgh to set up a Department of Crystallography. In 1962 he invited me and my family to go to Pittsburgh on a Fulbright Fellowship. Here I started my investigations into how crystalline Cl2 came to have its extraordinary herring bone structure. In 1963 we visited the University of Toronto and the Head of Chemistry offered me the post of Full Professorship. I accepted and we all had to return to England for one year to satisfy Fulbright conditions. We all went permanently to Toronto in 1964. In the new Department of Chemistry at Toronto I was given liberal office and lab space and substantial funds with which to get started. Automatic diffractometers were becoming greatly in demand and the manufacturers spared no efforts to get their products bought. In the end I decided on the four-circle Picker machine. This proved a good choice, the machine being operative for more than forty years. During the 23 or so years in Toronto the Picker had a variety of computers and devices for its full automation. We used the NRCVAX system of structure determination written by Eric Gabe and collaborators at the National Research Council Laboratories in Ottawa. We provided a structure-solving service for the Department and were engaged in other research activities. Our most exciting determination came early on, namely the discovery by A. D. Allen in 1965 of a ruthenium complex containing molecular nitrogen as ligand. Traditionally it had always been considered that molecular nitrogen was too inert to form chemical compounds. This work clearly damaged that tradition (8).
Overbutting of two nitrogen molecules in a ruthenium complex. From a study of the Cambridge Organic Crystal Database we made scatter-plots of non-bonded distances between identical atomic elements. We published the results later showing that for the halogens especially the effective van der Waals shapes are not truly spherical but flattened. (9) In 1974 a program BMFIT was written to give the closest match between molecules having some structural features in common. (10) We also collaborated on crystallographic matters with the Department of Pathology at Mt. Sinai Hospital in Toronto (11). During the 70's we were also approached by Esso to pursue crystal studies of normal alkanes. This interest has lasted ever since. Our first entry into these new studies was to discover that the published crystal structure of n-octadecane was wrong. This we corrected. We obtained some exciting results from our alkane studies. Mineral oils contain largely normal alkanes of many molecular lengths and when cooled this oil deposits waxes which greatly handicap the extraction of the oil. Despite enormous research effort by the oil companies the crystalline nature of these waxes was not known. We decided to examine a simple binary mixture of alkanes as a simple model of a wax. The C20:C22 phase diagram was examined by our Australian colleagues. It is quite complex and shows there are certainly new phases in the 50%:50% region. We obtained excellent crystals from such a 50%:50% mixture dissolved in dodecane. Weissenberg photographs were taken with the crystal cooled to 10°C. From these we inferred that the space group was orthorhombic Bb21m with a=4.99, b= 7.47 and c=56.26 Å., the two pure components being triclinic. We managed to find the crystal structure. Either of the two alkane molecules can occupy the same position their combined symmetry being a mirror normal to the z axis, a symmetry element which neither alkane possesses. This fascinating structure forms the basis of the alkane wax structures. At room temperature a disordered F phase is present. Later a diffractometric study was made at King's College after we had returned to U K of the binary pair n-docosane : n-tetracosane. The phase diagram is similar but is everywhere higher in temperature. A diffractometric study was made of the 50%:50% crystal. This confirmed our earlier results. A truly remarkable crystal structure containing molecules with twenty-four and twenty-six carbon atoms which shows twenty-seven Fourier peaks! (12)
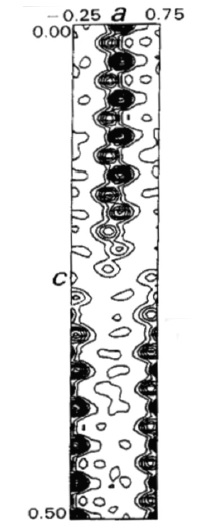
y=0 electron density map of the β-C24,C26, showing decreasing C-atom site occupancies towards the end of the C-atom chains.
Setting up the Picker at King's raised a number of problems particularly the change from 115V ac to 240V ac. However in all this I was greatly helped by a staff member, Dr Adrian Parkins. The Picker was newly fitted to a PC, an IBM 286, and Gabe's Fortran program for data collection was rewritten in Gbasic. The Picker behaved excellently and Adrian and I completed a large number of crystal structures for Staff members. In the early nineties King's acquired a Nonius CAD diffractometer. The Picker was subsequently devoted to further work with normal alkanes. Unfortunately, the Department of Chemistry at Kings College London was closed down permanently by the College authorities in 2006, and the Picker diffractometer destroyed. I am now Hon Sen Research Fellow at University College London continuing normal alkane studies.
References (1) G. A. Jeffrey, H. P. Koch and S. C. Nyburg, J. Chem. Soc. 1118, (1948) (2) E. G. Cox. Proc. Leeds Phil. Lit. Soc. 5 1-13 (1947) (3) D. W. J. Cruickshank, G. A. Jeffrey and S. C. Nyburg, Z. für Krist., 112, 385 (1959) (4) S. C. Nyburg, Acta Crystallographica, 7, 385 (1954) (5) J. F. D. Mills and S. C. Nyburg, Tetrahedron Letters, 11,1-3, (1959) (6) J. F. D. Mills and S C Nyburg J. Chem, Soc., 1485- 1463 (1960) (7) X-Ray Analysis of Organic Structures, S. C. Nyburg. Academic Press, 1961 (8) F. Bottomley & S C Nyburg, Acta Cryst., B24, 1289 (1968) (9) S. C. Nyburg & C H Faerman Acta Cryst. B41, 274- 279 (1985) (10) S. C. Nyburg Acta Cryst. B30, 251 (1974) (11) K. P. H. Pritzker, P-T. Cheng, M. E. Adams & S. C. Nyburg, J.Rheum., 5, 469-473 (1978) (12) A R Gerson & S C Nyburg, Acta Cryst., B50, 252- 256 (1994) |