- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
Memoir - James A. IbersMemoir | Publications | Curriculum Vitae | Videos | Slides | Articles | Obituary ACA Living History2020
To start at the beginning, I was born in Los Angeles and lived in California for the first 25 years of my life. Early on I wanted to become an archaeologist, then an astronomer, then a chemist. So I applied to Caltech, my neighborhood school for science and engineering, and was accepted after a grueling three-hour written examination. Before the first quarter of school began I was required, as were all 160-admitted Freshmen [no women in those days], to attend a one-week orientation camp on Santa Catalina Island. The most important message I took away was the Caltech Honor Code for all undergraduates. In its simplest terms: You can’t cheat in Science because you will eventually be found out. I have adhered to that Code as a husband, a father, a scientist, a teacher, a research director, and all others I have dealt with. I did surprisingly well academically in the first school quarter and thus was required to find a mentor who would supervise my research efforts. After talking to several Professors I chose to work both in the library and in the laboratory for Prof. Norman Davidson. Norm was interested in optical properties of metal ions in mixed oxidation states. After about a year Norm ran out of funding (National Science Foundation (NSF)), but he told me to see Dr. David Shoemaker of the Pauling group. I found a door in the basement of Crellin Laboratory with the name “Schomaker” on it.  Verner Schomaker Photograph by John A. Moore, University of Washington, courtesy of AIP Emilio Segrè Visual Archives. The office was dark, but I knocked and Prof. Verner Schomaker let me in. I told him I was an undergraduate looking for a lab position. Verner, who got his degree under Linus Pauling, was interested in electron diffraction of gases. The work originated around 1930 when very few interatomic distances were known. Verner sent me to one of his postdoctoral researchers, Ken Hedberg. I worked for Ken for several years. One day when I was talking to Verner in his always dark office, he mentioned that he was troubled by experimental evidence that UF6 was apparently unsymmetric, as this seemed to defy the Born-Oppenheimer approximation. By this time the Schomaker group had a French postdoctoral researcher, Jean Hoerni, who was trained a physicist. He and I calculated complex amplitudes for electron scattering. I don’t recall if we ever resolved the issue of the symmetry of UF6, but perhaps it was settled in a meeting we had with Prof. Richard Feynman of the Physics Department. In an ensuing discussion in Verner’s dark office he noted that the Fourier transform of the wave function is the atomic scattering factor (form factor). This was 1954 and I attended my first ACA meeting in Cambridge, MA where I found that I was presenting these results in the presence of Dr. G. W. Brindley whose form factors were in current use! I continued to attend ACA meetings until they got gobbled up by the macromolecular types. I find specialized meetings more useful, an example being Journées des Actinides, a small European meeting that brings together chemists and physicists who are interested in the actinides. It is amazing how little chemistry most theoretical physicists know!  J. Holmes Sturdivant Photograph courtesy of the Archives, California Institute of Technology. The undergraduate curriculum at Caltech was fixed by your Deity and Caltech President Prof. Robert A. Millikan. As a result I took an “elective” course in X-ray crystallography from Prof. J. Holmes Sturdivant. These days you can’t get near an X-ray beam on a diffractometer; in those days we centered the crystal in the collimator with mA and kV turned down. Unit cell determinations were usually obtained from a Laue camera but the instrument of choice for data collection was a Weissenberg camera. A Buerger precession camera was available, but was rarely used. Estimation of intensities was done visually, by packing the Weissenberg camera with multiple photographic films separated by very thin Cu sheets. As an intensity standard I made up a film strip from multiple beam exposures. The material I chose to examine was ceric iodate monohydrate, probably because its crystals were a pretty yellow color! It was not the wisest choice for two reasons: it had a large unit cell for those days and it was anhydrous until Don Cromer at Lawrence Livermore Laboratory identified a water that I had missed. In any event I was hooked on crystallography. I estimated intensities at night while listening to records of Mozart piano sonatas as played by Wanda Landowska. Verner and his associates had realized the “B” in IBM stood for Business and they devised the “M-card system” for calculating Fourier syntheses that depended on IBM sorters, tabulators, and mergers. These devices were on the second floor of Throop Hall and were used by the Caltech Business Office. An arrangement was worked out for me to use these devices at night. Except for the occasional moth getting caught in the tabulator the calculations went smoothly. I was able to calculate a three-dimensional Fourier synthesis of ceric iodate. Failure to find that it was actually a monohydrate was perhaps the first, but certainly not the last crystallographic error I would make. Speaking of errors reminds me of Dick Marsh, another member of the Pauling group. Dick’s main pastime, when he was not working on a project for Pauling, was finding errors in the literature. The victims were “Marshed." I eventually got “Marshed."
 Dick Marsh and Linus Pauling. For my graduate studies I applied to and was accepted by the University of California, Berkeley, and Caltech. (Remember as an Angelino it was my impression that there was nothing much East of the Sierra Nevada Mountains, including Ivy League schools!) Ken Hedberg urged me to go to Berkeley as going to the same graduate school as one’s undergraduate school was strongly discouraged. For personal reasons I chose Caltech.
1955-1961: Shell. With Verner’s help I had secured employment in Dave Stevenson’s group at Shell Development Company in Emeryville, California. Emeryville is just across the Bay from San Francisco. Shell was interested in better characterization of solids and that was where I fit in. Overall the group was concerned with testing new instrumentation to prevent other Shell laboratories from buying instruments they did not need. My colleagues in Dave’s group were very generous with their time: Jerry Swalen taught me to program in FORTRAN; Bob Snyder et al. devised the use of oil to facilitate the X-ray examination of air- and water-sensitive crystals. Ed Smutney showed us the wonders and apparent sins of North Beach in San Francisco! In my spare time I wrote a number of crystallographic papers: among others, estimates of standard deviations of observed structure factors and of the electron density from intensity data, a variety of new atomic form factors, including relativistic ones, anharmonic oscillations of nuclei, and tables of atomic scattering amplitudes for electrons (in volume 3 of International Tables for X-ray Crystallography (1962)). This last effort was co-authored in 1959 with Boris Vainshtein of the Soviet Union and was carried out on an IBM computer available at IBM San Francisco. In 1959 the U.S. was engaged in a “Cold War” with the Soviet Union! Dave Templeton at UC Berkeley noticed my crystallographic papers and asked me to apply for an assistant professor position at Berkeley. I told him “No” as I was very happy at Shell. Shortly thereafter Shell at Emeryville fell apart as a result of a reorganization at Royal Dutch Shell. It is interesting to contemplate what might have ensued if I had said “Yes” to Dave Templeton!
Walter Hamilton
I did not like some of the computer programs in vogue at that time, as one was forced to refine on Fo, weights were difficult to assign for small Fo, and there was no attention given to Fo < 0. Verner had taught us that one never changes the data but only the model. Fo2 is far closer to the data than Fo, and fortunately the ORFLS least-squares program available from Bill Busing and Henri Levy of Oak Ridge National Laboratory allowed one to refine on Fo2. Thus I put together a suite of programs and the necessary instructions for ORFLS, ORTEP, Carroll Johnson’s thermal plotting program, and FORDAP, the Fourier program from Al Zalkin at Lawrence Berkeley National Laboratory. These all compiled nicely on the Brookhaven CDC 3600 computer. One cannot say enough for the great contributions the National Laboratories have made to crystallography, especially with the availability of open source software. [As a historical note, Edward Hughes in the Pauling group in 1941 was the first to apply the least-squares technique to the refinement of crystal structures.]
"The Red Books." Around 1961 Kathleen Lonsdale became General Editor for a projected four volume revision of International Tables for X-ray Crystallography. Overall, Caroline MacGillavry and Gerard Rieck were in charge of Volume III, Physical and Chemical Tables, that included X-ray and electron diffraction scattering factors and a variety of largely mathematical details. [Some of you may remember “The Red Books” with their lousy bindings.] Caroline asked Walter Hamilton and me to edit Volume III. It was there that the genesis of the book Hydrogen Bonding in Solids by Hamilton and Ibers (1968) occurred. I took the opportunity to express to Caroline my interest in drawings by M. C. Escher, a fellow Dutchman. She provided contact information. Escher was taken by surprise and indicated that he never sold fewer than four prints at a time. Some of you may remember the prints I ordered as they have hung in all of our residences. “Day and Night” remains my favorite and is currently hung in my bedroom. I don’t remember why I was there but around 1962 I attended a meeting of about 12 people in the French Alps overlooking Lake Geneva. In attendance were Martin Buerger, Jose and Gabrielle Donnay, and several high-level theorists, including Hans Wondratschek. The purpose of the meeting was to organize the space group information for the new International Tables. I, as the “pci” — the practicing crystallographic idiot — made two important contributions. I kept the theorists from making “c” the sole monoclinic symmetry axis and I had them include the necessary information to define a unique unit cell. This helped prevent crystallographers who collected limited data sets from collecting half the data twice. Incidentally, the “b” axis as the symmetry axis, was chosen by the mineralogist Grose in the 18th century who characterized diverse crystals and provided their axial ratios. 1965: Northwestern University. Although I was very happy at Brookhaven it was situated in the middle of nowhere with nothing much but duck farms to the East of the Hamptons. Our lovely two-acre lot and home in Bellport were great but our dog joined packs and attacked the postman and we dared not let our cat outside. Also, setting up a laboratory at Brookhaven to do preparative chemistry would have been difficult. Although to the West was New York City with all its attractions, getting there involved The Long Island Railroad, where I use the term “railroad” euphemistically. Joyce and I were very interested in cultural events, especially the theatre. Thus when I was approached by Iowa State University as a possible replacement for Bob Rundle, who was retiring, I was tempted, but Ames was not my idea of an environmental improvement. [Larry Dahl took the Ames offer.] Somehow Northwestern got wind of the possible Ames offer and countered with one of their own. Evanston, and especially Chicago, were civilization and a full-professor offer from Northwestern was an easy choice. As this is an “ACA Living History” I have up to this point concentrated on crystallographic matters, although I warn you that I do not consider myself to be a crystallographer but rather an inorganic chemist. To emphasize this point, since joining the faculty at Northwestern about 9% of my publications have been in Acta Cryst., whereas about 33% have been in Inorg. Chem.
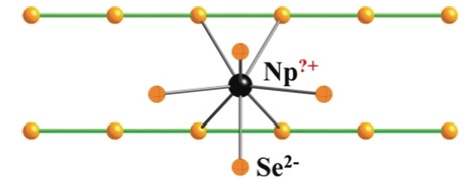
As part of my start-up package at Northwestern I purchased a brand-new four-circle Picker diffractometer. For computing, Northwestern had a CDC 3600 computer so I simply brought the program suite I had at Brookhaven and compiled the programs at Northwestern. If more computing power were needed we had overnight access to the CDC 7600 at Lawrence Berkeley Laboratory. The Picker was controlled by punched cards and then paper tape. To collect data efficiently I wrote something akin to the traveling salesman problem. I was fortunate to have an electronics shop available to fix encoder problems. We devised a cold stream to cool crystals. It worked reasonably well, but far better when Jean-Jacques Bonnet, a postdoctoral researcher, supplied a glass transfer tube from his glassblower in Toulouse. Overall this setup for the Picker worked very well probably for about 20 years, but was quickly abandoned by my group once the Nonius version of a four-circle diffractometer became available. As I had one of the few diffractometers in 1965 I collaborated with a number of chemists to solve a variety of structural problems. It was evident to me and to Larry Dahl that solving the crystal structure was the fastest way to characterize a compound. This fact was later “discovered” by Al Cotton. Concomitantly my research group began to produce compounds often involving phosphines, especially triphenylphosphine. Common sense dictates that if you know something better than you can determine it crystallographically you should make use of it. This led me to write a group refinement program for phenyl groups. It also led my group into hydrides, molecular O2, molecular N2, NO, SO2, CS2, and aryldiazo compounds, among others. In the United States research projects are driven by available funding. The research detailed above was largely funded by the NSF. But our interest in molecular oxygen compounds enabled me to secure National Institutes of Health (NIH) funding. The ensuing research was largely organic chemistry! The group made a variety of porphyrins, delved into porphyrin oxygen chemistry, made synthetic analogues of known protein molecules, and began an ongoing collaboration with Dick Holm on iron-sulfur chemistry. The research on iron-sulfur chemistry led my group to the very rich chemistry of soluble metal chalcogen anions (NSF), examples being the [AuTe7]3− anion and the [(Te4)M(μ−Te4)M(Te4)]4− anions, M = Cu, Ag. In the last decade my interests have turned to solid-state chemistry, in particular to research into the solid-state chemistry of the actinides (U and Np). This research was supported by the Department of Energy - Basic Energy Sciences (DOE) and comprised my support from about 2007 until I elected to cease accepting new graduate students. However, it continues in collaboration with two former postdoctoral researchers, Adel Mesbah (France) and Jai Prakash (India). An example of this work is “NpSe2: a New Binary Chalcogenide Containing Modulated Selenide Chains and Ambiguous-Valent Metal”, which was published recently in Angewandte Chemie.
Overall it is a fun journey through a plethora of problems and curiosities. My many students, postdoctoral researchers, visitors, and research collaborators continue to make this possible. To them I dedicate this History. Jim Ibers
Editor’s Note: Among the Awards and Honors Jim Ibers has received are the American Chemical Society Award in Inorganic Chemistry, American Chemical Society Award for Distinguished Service in the Advancement of Inorganic Chemistry, the Linus Pauling Medal, the California Institute of Technology Distinguished Alumni Award, and the Martin J. Buerger Award of the American Crystallographic Association. He has been elected to the American Academy of Arts and Sciences and to the U. S. National Academy of Sciences. |