- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
Memoir - Abraham ClearfieldMemoir | Publications | Curriculum Vitae | Videos | Slides | Articles | Obituary
A Life in Crystallography Abraham Clearfield 2013
Shortly thereafter the great depression arrived and we along with others were hard hit. My father had a pushcart stand in a market selling fruit and vegetables. Our part of South Philadelphia was divided into sections. The Irish lived from the Delaware River to Fourth Street. The Jewish neighborhood extended from Fourth to Eighth Street and beyond that for as far as the eye could see was the Italian section. There was some overlap so we had two Italian families and one Irish whose boys were readily accepted into our gang. White people lived in the broad streets while the narrow streets were mainly African American.
When I was 12 years old my brother Ted gave me a Chemcraft chemistry set for my birthday. It was the best possible gift and started me on the road to being a chemist. A very poor high school chemistry course did not dissuade me. My high school, Southern High, was an all boys' school that reflected the mix of nationalities and religions. Race or religion did not bother me; I made friends with all groups. I was elected secretary-treasurer of my graduating class, a post I held later in the U.S. National Committee for Crystallography, 1995-97. Although I finished 12th in a class of 400 I did not get a scholarship as there were very few in those days. However, Ted came to the rescue. He was in the Navy and stationed in the South Pacific. Every month he sent me part of his pay and together with money I earned in a variety of jobs in high school it was enough to see me through with a B.A. from Temple University. The chemistry department offered me a position to run the physical chemistry lab in their night school and earn an M.A. with free tuition and a monthly stipend. Half way through my M.A., I married Ruth and after some reflection she convinced me to go on for a Ph.D. I was accepted at Rutgers University and did so well in the entrance exams that I was offered a fellowship with Philip A. Vaughan. Thus, began my career in crystallography.
Phil had received his Ph.D. at Caltech under Linus Pauling. My project was to determine the structures of zirconyl chloride, ZrOCl2·8H2O. This compound was important because pure zirconium metal was required for cladding of nuclear reactors but very little zirconium chemistry was known at that time. The zirconyl compound was the major source of a soluble zirconium compound.
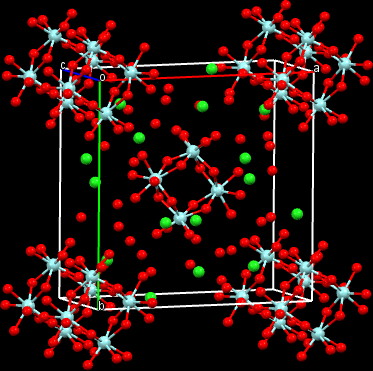
We had no automated equipment and no computers. We used a Weissenberg camera to obtain films of the individual layers, read the spots by eye and calculated structure factors by hand. Pauling punch cards were used for electron density calculations. If that were not stressful enough the crystals lost water in air with destruction of the crystals. They had to be kept in sealed capillary tubes with some mother liquor in the tube. I solved the structures of the chloride and the bromide and they are nothing like what had been predicted (Figure 1).
Figure 1. A three dimensional representation of the structure of zirconyl chloride,
The compounds are tetramers with the eight-coordinate Zr4+ ions at the corners of a square. There are four >OH-groups bridging Zr ions. The coordination is completed by four water molecules bonded to each Zr. The eight halide ions surround the tetramer yielding the formula [Zr4(OH)8·16H2O]8+ 8Cl‾. This study showed that there were no ZrO2+ ions in the solid and this has proved to be the case for the solutions also. Interestingly, a group at Argonne National Lab and Northwestern University has recently begun a study of the structure of zirconium ions in solutions containing sulfate ion. They have used an array of the latest scattering techniques to attempt to determine the nature of the ions in solution as opposed to the structures that crystallize from those solutions. This paper referred to our work on the zirconium tetramer and my subsequent studies on the zirconium sulfates. So my 1956 paper in Acta Crystallographica [A. Clearfield, P. A. Vaughan, 9, 555] is still of interest more than 50 years later.
Phil wanted me to present my study at the upcoming ACA conference at the University of Michigan (in June, 1953). Lucky me, this was the meeting where Hauptman and Karle presented their "direct methods" procedure. Everybody who was anybody in crystallography stood up to criticize the presentation but Phil leaned over and whispered to me, "These guys really have something." He even solved a structure using direct methods and after some editorial problems was able to publish it.
Upon graduation I took a job with the Army Quartermaster Corp., in Natick, Mass. My job was to develop X-ray data of stretched fibers. I had an old G.E. generator with a rabbit ears tube. The tube needed adjustment, so when the technician came he showed me how to correct the problem. He held the tube by the rabbit ears, loosened the screws and made the adjustment. Then he said, "Make sure all the screws are tight before you let go." Then he let go and you guessed it, it struck bottom and smashed into a thousand pieces. With no tubes available and no possibility for a new X-ray generator I left for an industrial job with Magnesium Electron in Niagara Falls. Among their major products were semiconductor powders BaTiO3 and SrTiO3 prepared for the transistor industry. Often they made a batch that did not function properly. I convinced the physics group to purchase a powder X-ray unit so we could examine the powders during preparation and ensure the correctness of every batch based on crystallographic principles. As a side issue I solved the structure of BaTiS3 and the Ca and Sr sulfides using X-ray powder data [Acta Cryst. 16, 135 (1963)].
Somewhat later I taught a course in crystallography in the evening at Niagara University. I drew about 15 students working in industry and was astonished to find that none of the companies had X-ray equipment even though they made solid products based on clays and cement. I tried to teach them what could be learned using X-ray powder diffraction. A student, Jim Stynes, who worked in our analytical division obtained permission to use me as his research director at Niagara University so he could qualify for a master's degree. Working together we synthesized crystalline zirconium phosphate, Zr(O3POH)2·H2O (α-ZrP) a layered compound that behaved as an ion exchanger [A. Clearfield, J. A. Stynes, J. Inorg. Nucl. Chem. 26, 117 (1964)]. Although the company patented this compound, they were not going to develop it further. I had acquired a liking for teaching and wanted to further develop this class of layered compounds. Of course the key was structure so I wanted a place where I could have an X-ray lab.
I obtained a position with Ohio University in Athens, Ohio where I was able to buy an X-ray generator and Weissenberg camera. In short order, two students, G. David Smith and Robert H. Blessing, who wanted to study crystallography, joined my research group. I instituted a course in X-ray Diffraction and Crystallography with lab sessions on use of the equipment. Dave solved the structure of α-zirconium phosphate and Bob worked on new Zr phases and structure studies. Then Bill Duax joined my group as my first post-doc. With these three jokesters, every day was a happening. Bill did his usual acrobatics to the delight of the females in our group. Yes, I encouraged women to be a part of the group in 1964.
Dave loved to catch flies and paste a tail on its rear. The fly would lift off and remain suspended in air unable to fly freely and the boys would try to knock it down with a squirt bottle from five paces back. Our initial labs were in the basement of the chemistry building. One morning I entered the lab to see Dave standing on a box looking out at girls walking past our parking lot. Bob was at the air hose on the lab bench. At Dave's signal he opened the air jet to blow air but where? They hooked up tubing to the rear of a parked car and aimed at the passing girls. They took turns changing places.
Bill and I tried to solve structures from powder data by cutting out the printed peaks and weighing them for intensity data. We did find the heavy atoms but without digital equipment and computers we could not do any meaningful refinement. Bill did produce a major paper on the ion exchange mechanism of Na+ exchange in zirconium phosphate [J. Phys. Chem. (1969) 73, 3424-3430].
During this time Sir William L. Bragg came to speak at Georgia Tech in Atlanta. This was an event not to be missed. Bob, Dave and I drove over the hills of West Virginia to hear this founder of X-ray crystallography and shake his hand. I still remember the great man's final statement. "I have no doubt that someday we will solve the structures of proteins routinely and this will revolutionize our understanding of biological processes."
Jobs were very difficult to obtain at this time so when Bill was invited to an interview at the Medical Foundation of Buffalo. I urged him to go even though he was skeptical. Well, he took the job and the rest is history. Imagine my pride when Bill became President of the ACA and later Chief Executive Officer from unheralded Ohio University. Bill to me was a kindred soul, the care of his family and his devotion to teaching young students pleased me no end. I was awarded an endowed lectureship for my service as Associate Dean of the College of Science at Texas A&M. After having two Nobel Laureates and several world-renowned chemists as speakers, I invited Bill. His talk was eclectic as expected. In true fashion he used the honorarium for science mentoring of high school students. What better thanks could a teacher have?
My next student that was crystallographically inclined was Jan Marshall Troup. He learned to do crystal structures with me but then went to Texas A&M to work for Frank Albert Cotton. Cotton's lab contained a completely automated X-ray unit which allowed Jan to solve all the problems Cotton had brought unfinished from MIT. Jan won the award for the best dissertation from the College of Science upon graduation. Then Jan and Bert Frenz founded Molecular Structure Corporation, the first company to do crystal structures for toll. I was on the Board of Directors for about ten years.
You see, Al Cotton and I had been classmates at Temple University. So just after Jan graduated I was invited to apply for a position at Texas A&M and accepted their offer. This was 1976 and I am still here. Meantime Bob and Dave had obtained post-docs and as fate would have it they eventually both joined Bill at the Medical Foundation of Buffalo. A few years later Herbert Hauptman was made Director of Research of the Medical Foundation and the name was changed to the Hauptman Woodward Medical Research Institute. So I was invited to present a lecture at the Institute and Herb and I became good friends as did Ruth and Edie. Ruth liked the ACA the best of all the meetings we attended. She had a large cadre of friends who every year looked forward to enjoying each other's company at these conferences.
Abe and Ruth at the Buffalo ACA meeting (1999).
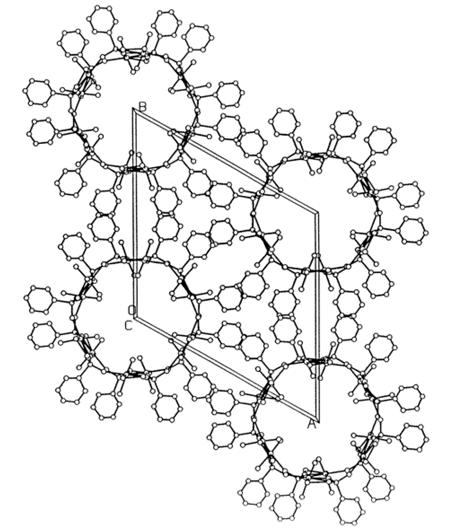
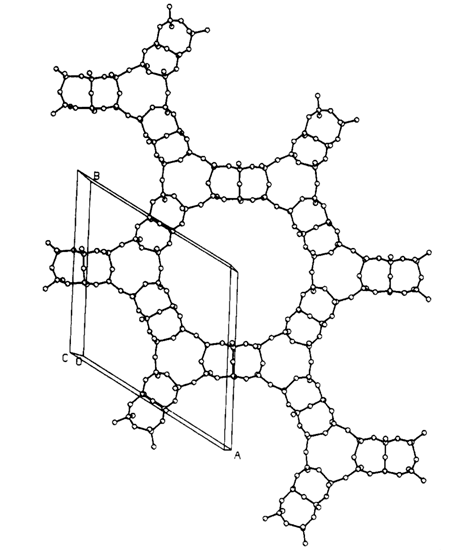
At Texas A&M I was able to build a major X-ray diffraction laboratory and I was instrumental in hiring Joe Reibenspies and later Nattamai Bhuvanesh to manage this lab. We also rekindled our interest in solving crystal structures from X-ray powder data and this time we were successful [P. Rudolf, A. Clearfield, Acta Cryst. (1985) B41, 418]. Figure 2 is an example of the type of structures we were able to solve. At one time we even held the record for size, doing a 50-atom problem from powder diffraction data [Inorg. Chem. (1996) 35, 1468]. A notable success was to unravel the structures of the largest (at that time) zeolite pore structures. They were designated H1 and VPI-5 and could not be differentiated. Both compounds had space group P63cm with very similar unit cell dimensions. We found that in H1 the six membered rings were disordered, so had special arrangements whereas the VPI-5 has ordered six membered rings (Figure 3) [D. M. Poojary, J. O. Perez, A. Clearfield, J. Phys. Chem.(1992) 96, 7709]. Incidentally my former student synthesized these compounds as a post-doc at VPI (Virginia Polytechnic Institute and State University).
Eventually, I was able to convince the ACA governing board to create an X-ray Powder Special Interest Group, which I chaired in 2004. In between I was Chairman of the Synchrotron Radiation Special Interest Group 1995-96, Secretary/Treasurer, U.S. National Committee for Crystallography 1995-97, Vice President and President of the ACA 1998, 1999. I also won awards for excellence in research by the Southwest (1995) and Northeast (2008) sections of the American Chemical Society.
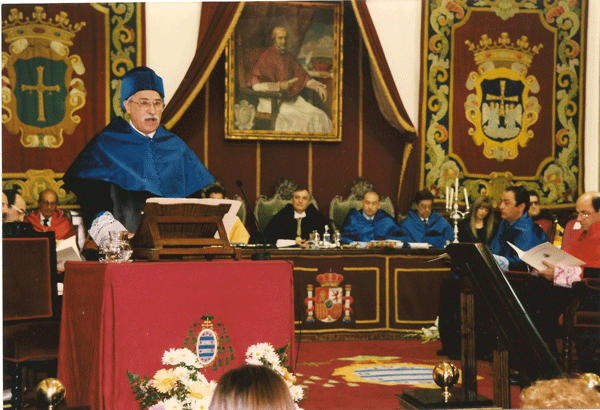
Abe receiving honorary degree from the University of Oviedo, Spain (1998).
In conclusion it should be noted that we did crystal structures for students in foreign countries like India, Iran and Nigeria as a goodwill gesture. I also received an honorary Ph.D. (Honoris Causa) from the University of Oviedo, Spain, March 18, 1998 for introducing their chemistry department to the intricacies of metal phosphonate chemistry. In recent years the teaching of crystallography has changed. With the advent of a Materials Science Program, which I recommended as Associate Dean for Research in the 1980s, but has only been a reality at Texas A&M in the past three years, the emphasis is on powder diffraction rather than structure solutions. I still give advice on structure solutions to the chemistry students and intense training to my graduate students but diffraction theory is now missing from the X-ray course.
It has been a highly interesting and rewarding life in chemistry and crystallography but it has not yet ended. The zirconium phosphates have continued to interest scientists around the world with more than 10,000 papers and more each year. We are currently using them as nanoparticles for anti-cancer drug delivery. The search for knowledge and wisdom is eternal.
Abe Clearfield |

 My parents were born in Ukraine, then a part of Russia. My family lived in Romanivka, a small town near Kiev. My father had a horse and wagon with which he collected produce from local farmers to sell in Kiev, ten kilometers away. He slept under the wagon, even in winter, to sell the produce in the morning. My father emigrated to the US in 1912 but because of WWI and the subsequent revolution and upheavals it took my mother nine years before they were reunited, having lost three children in the interim. My mother was able to bring out only one child, Sam, who was 15 years my senior. The two girls died from the flu in a refugee camp on the Rumanian-Ukraine border. The boy, Tevia, was killed in a bandit raid. I am not sure but think he was trying to protect the girls. My mother substituted her nephew using Tevia's passport. So in all there were three boys, Sam, Ted and me the baby. The nephew, Harry, went off on his own. My brother Ted and I were born in Philadelphia, PA.
My parents were born in Ukraine, then a part of Russia. My family lived in Romanivka, a small town near Kiev. My father had a horse and wagon with which he collected produce from local farmers to sell in Kiev, ten kilometers away. He slept under the wagon, even in winter, to sell the produce in the morning. My father emigrated to the US in 1912 but because of WWI and the subsequent revolution and upheavals it took my mother nine years before they were reunited, having lost three children in the interim. My mother was able to bring out only one child, Sam, who was 15 years my senior. The two girls died from the flu in a refugee camp on the Rumanian-Ukraine border. The boy, Tevia, was killed in a bandit raid. I am not sure but think he was trying to protect the girls. My mother substituted her nephew using Tevia's passport. So in all there were three boys, Sam, Ted and me the baby. The nephew, Harry, went off on his own. My brother Ted and I were born in Philadelphia, PA.