Memoir - Philip Coppens
Memoir | Publications | Curriculum Vitae | Videos | Slides | Articles | Obituary
The Old and the New: My Participation in the Development of Chemical Crystallography during 50+ years
Philip Coppens, Chemistry Department, University at Buffalo, Buffalo, NY
Phys. Scr. 90 058001 doi:10.1088/0031-8949/90/5/058001
Reproduced with permission from The Royal Swedish Academy of Sciences.
|
Highlights of my scientific experiences during a golden age of science in which new avenues were opened due to an unprecedented increase in experimental and computational facilities are summarized.
|
 My Scientific Experience over the Decades. I started in Crystallography under Prof. Caroline McGillavry at the University of Amsterdam after majoring in Physical Chemistry in the mid nineteen-fifties. I was attracted by the beauty of crystals and their periodic arrangement, the mathematical aspects and the fact that crystallography unlike some other physical methods could produce unambiguous results. It was a time not long after World War II, when everybody was excited about the new possibilities in Science while career possibilities were less of a consideration, the possibilities seemed endless. It also was the time of Beevers-Lipson strips, Hollerith punch-cards used at primitive computers not located in the University, and two- dimensional projections. McGillavry was a whiz at maximizing the use of all those while applying a great deal of intuition. Several times I saw her deduce the coordinates of a bromine atom from the intensities on a Weissenberg photograph. X-ray safety and other precautions were still in their infancy. A superbly capable technician had an X-ray burn on his wrist. A cat in the lab had the dexterity to climb on a closet in the X-ray lab where the poor animal was electrocuted by an exposed high voltage lead. Many visitors were passing through the laboratory in Amsterdam. My Scientific Experience over the Decades. I started in Crystallography under Prof. Caroline McGillavry at the University of Amsterdam after majoring in Physical Chemistry in the mid nineteen-fifties. I was attracted by the beauty of crystals and their periodic arrangement, the mathematical aspects and the fact that crystallography unlike some other physical methods could produce unambiguous results. It was a time not long after World War II, when everybody was excited about the new possibilities in Science while career possibilities were less of a consideration, the possibilities seemed endless. It also was the time of Beevers-Lipson strips, Hollerith punch-cards used at primitive computers not located in the University, and two- dimensional projections. McGillavry was a whiz at maximizing the use of all those while applying a great deal of intuition. Several times I saw her deduce the coordinates of a bromine atom from the intensities on a Weissenberg photograph. X-ray safety and other precautions were still in their infancy. A superbly capable technician had an X-ray burn on his wrist. A cat in the lab had the dexterity to climb on a closet in the X-ray lab where the poor animal was electrocuted by an exposed high voltage lead. Many visitors were passing through the laboratory in Amsterdam.
One of them was Gerhard Schmidt who had just started to set up the first X-ray laboratory in Israel at the Weizmann Institute of Science. Gerhard had the vision that X-ray Crystallography was more than solving crystal structures and should be used to solve chemical problems. This was unusual at a time during which the main effort was directed to solving the phase problem. I was fascinated. McGillavry and Schmidt came to an agreement under which I would do my PhD research at the Weizmann Institute and return to defend my PhD thesis in Amsterdam. My partner and I married and we moved in 1956. I found a very lively group in the Crystallography lab at the WI (Fig 2), including Mendel Cohen (third from left) and later Fred Hirshfeld who returned from a postdoc with Bill Lipscomb in the US. The research involved photochemistry, the study of light-induced processes in crystals, now referred to as photocrystallography, a term I coined much later in 1997 upon returning to the field [1]. In the absence of lasers, which had not been invented yet, the light source was the sun, as described by Fred Hirshfeld 'conveniently installed at 92,955,807 miles above the roof of the Weizmann Institute'.

Gerhard M. J. Schmidt
Even then the scientific and technical advances of the previous decade were impressive, so much that Gerhard described the earlier period in which conclusions could not be based on known crystal structures as the heroic era, a term which now equally could be applied to the period I was just describing.

Crystallography at the Weizmann Institute in early 1957.
It could be described as a second heroic era employing, with the benefit of today's perspective, incredibly primative tools. There was also the beginning of X-ray charge density analysis, led by the inspiring work of Fred Hirschfeld, in which I participated.

Fred Hirshfeld, 1969 IUCr Congress. http://www.iucr.org/gallery/1969/iucr-viii
It all amounted to the application of Crystallography to Chemical problems, following the vision of Gerhard Schmidt. This remained the guiding light of my future work and that of others being trained in the Weizmann laboratory including Leslie Leiserowitz, Dov Rabinowitz, Meir Lahav and those who followed.
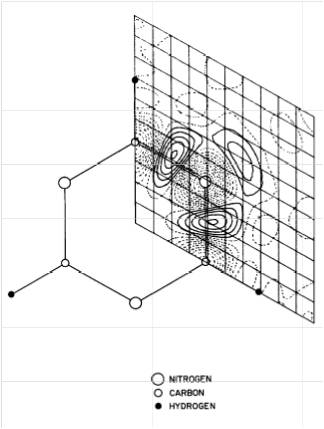
The first X-N difference map in which spherical neutron parameters locations are subtracted from the experimental density of s-triazine.
From: reference 6, Science 158 1577-1579. Reprinted with permission from AAAS
After completing my PhD research my family and I spent a very pleasant summer in the Netherlands during which I defended my Ph. D. based on the difference between the photochemical properties of two polymorphs of p-nitrophenol, one of which turned dark red on light exposure without changing the shape of the original crystals, even though their content became amorphous and insoluble, while the second polymorph was fully light-stable. During that period I had the privilege of attending my first IUCr Congress which took place in the UK in Cambridge in 1960 and was attended by many of the founding greats of Crystallography. I met for the first time an incredibly active and mathematically skilled Walter Hamilton, who became my boss during a following two year postdoctoral appointment at Brookhaven National Laboratory. One of the events I will not forget was not crystallographic but involved meeting the graphic artist M.C. Escher who was selling his original gravures for very reasonable prices, an opportunity I regretfully had to pass up as we were on our way to the US and could not accommodate more luggage. Brookhaven Laboratory was in its early glory days, especially in nuclear chemistry, but also strongly advancing in crystallography. It gave enormous freedom to young postdocs who could essentially choose their own research topics. Of particular interest was the graphite-reactor neutron source which allowed collecting data for structure determination independent of assumptions about atomic form factors. I spent many weeks at the reactor collecting such data. After two years I returned to the Weizmann Institute as a staff scientist, collaborating with Fred Hirshfeld on charge density analysis, and with Leslie Leiserowitz on accurate evaluation of X-ray absorption corrections by numerical Gaussian integration, the latter resulting in a heavily cited paper [5]. The program was adapted by a commercial vendor, who unfortunately introduced a few mistakes which resulted in a number of phone calls to an innocent me.
I resumed my work at BNL in 1965, where in the meantime the graphite reactor had been replaced by the High-Flux-Beam Reactor (HFBR). It was commissioned in December of that year and dramatically increased the power of neutron diffraction. It allowed an accurate comparison of X-ray and neutron diffraction results which opened up an incredible research opportunity. Somewhat surprisingly at that time, X-ray anisotropic temperature factors from room temperature data on crystals of the simple molecule s-triazine showed considerable positive bias compared with the neutron values the former being larger in directions clearly suggesting shortcomings in the spherical-atom scattering factors used in the X-ray experiment. Construction of the first X-ray-Neutron difference map (Fig. 4) confirmed this interpretation, showing lone pair electron density at the back of the nitrogen atoms and bonding density in the C-N bonds [6]. Further work showed that such bonding features were quite efficiently absorbed in the popular least squares refinement of X-ray data with spherical-atom form factors. The work helped to silence the skeptics who consistently described the small band of upstarts active in X-ray Electron Density Analysis somewhat derisively as 'electron seers'. I worked on the elimination of multiple reflections in crystals, and with Walter Hamilton on extinction theory and on errors in electron density maps [7,8] . The work attracted the attention of David Harker, then at his own Crystallographic Institute at the Roswell Park Memorial Institute in Buffalo, who had been asked by the Chemistry Department of the State University of New York at Buffalo to recommend a candidate for a Faculty position, with the intention to establish a Crystallographic Laboratory in the Department. As Buffalo was (and still is) a center of crystallographic activity, and I wanted to return to a University environment to teach and build up my own research group, I accepted, a decision I have never regretted. So I and my family, which by then included three sons, moved to Buffalo to a rapidly expanding Chemistry Department.
At the same time the field of Charge Densities started to develop. In particular Robert Stewart at Carnegie-Mellon was a prime mover in the field. He determined the first molecular dipole moment from X-ray data, of uracil [9] and with Davison and Simpson derived a bonded hydrogen atom scattering factor from the theoretical charge density of the hydrogen molecule [10], a result much used by crystallographers in the following years. Stewart pioneered the introduction of spherical harmonic functions in the analysis of charge densities [11]. Others in the small but increasing group of enthusiasts were Ted Maslen from Perth, Australia, Dirk Feil from Twente, the Netherlands, Kurki-Suonio from Helsinki, Finland and Finn Krebs Larsen from Århus, Denmark and not to forget Fred Hirshfeld, whose many contributions are still widely applied not only by crystallographers, but also by theoretical chemists. Hirshfeld charges have become a widely accepted concept. With Niels Hansen, a bright student from Finn Larsen's lab we developed and coded a version of the multipole refinement method, in which the electron density is fitted by radially flexible atom-centered spherical harmonic valence density functions. The method was published in 1978 [12] and is still widely used. It has recently been extended to include core density radial changes. In parallel we worked on the improvement of the accuracy of experimental X-ray intensities, following the earlier work with Leslie Leiserowitz, in a close collaboration with Pierre Becker, visiting from France, whose mathematical skills and physical insight were indispensable, on the theory of isotropic and anisotropic extinction in crystals [13,14,15], both being widely applied in subsequent years.

With Mogens Lehmann (middle) at the 10th IUCR Congress in Amsterdam 1975.
In 1974 my family and I returned to Europe to spend a year in France, where I served a very fruitful period at the French-German-English Institut Laue-Langevin built around a high- flux neutron reactor, and at the CNRS in Grenoble. A number of lifelong friendships resulted from this stay, one of which is illustrated in Fig. 5. At this time an increasing trend became comparison of the experimental electron density results with theoretical densities. The first stumbling block to be overcome was the thermal smearing implicit in the experimental density maps. With Ed Stevens and John Rys we developed a method to thermally smear theoretical densities [16]. It was applied to data on crystals of formamide, but later replaced in X-ray charge density work by plotting the static multipole functions obtained by refining thermal motion separately in the refinement, thus achieving in principle a deconvolution of charge density asphericity and thermal motion. Such static deformation density maps and the corresponding second derivative Laplacian function derived from the total density introduced in the field by Richard Bader. Bader's great contribution was the 'Atoms in Molecules' theory, which emphasizes the prime importance of the total electron density [17]. It has become a prime tool in the analysis of charge densities. The theory has been applied in the definition of several informative new functions as described by Carlo Gatti in this issue [18]. An interesting interlude occurred when Vaclav Petricek the Institute of Physics of the Czechoslovak Academy of Sciences, who had published a number of articles with a strong mathematical content, joined our group as a Postdoctoral Fellow. At the time I was intrigued by the low temperature metal-to-insulator transition of tetrathiafulvalene- tetracyanoquinodimethanide (TTF-TCNQ) which was accompanied by the appearance of incommensurate reflections, interpreted as dimer formation in the homogeneous stacks of TCNQ molecules. How large were the displacements which caused this dramatic change in properties? I suggested to Vaclav that he looked into the analysis of incommensurate modulated structures. He very quickly excelled, and since has become the world expert in the field who is with his collaborators continuing to develop the program JANA, which has become very widely used. With the new program we derived the (small) molecular shifts within both stacks of TTF-TCNQ using synchrotron measurements collected at the National Synchrotron Light Source at Brookhaven National Laboratory [19] and also applied the equations developed to the modulations in the high Tc superconductors Bi 2Sr2−xCaxCuO6 and Bi2Sr2−xCaxCu2O8 [20] and other structures.
As the charge density field developed, conferences increased in importance. The first were initiated by the unforgettable Richard (Dick) Weiss, who, working at Watertown Arsenal in Massachusetts in 1966, got a call from the Army, that a Conference center in the Adirondack Park in Upstate New York had been reserved and needed a conference. This was the beginning of the Sagamore conferences on Charge Spin and Momentum Densities which became triennial, number XVIII taking place in 2015 on the Italian island of Sardinia. It was on returning from the 1976 Sagamore meeting in Finland, during a discussion on the ferry from Finland to Sweden, that Vedene Smith (Fig. 6) and I decided to apply for a Gordon Conference on Electron Distribution and Chemical Bonding, the first of which we co-chaired. It took place in 1978 at Plymouth State College in New Hampshire and was followed by many others (Fig.7) and continuing. Smith, of Queens University in Kingston Ontario, was the first theoretician who actively participated in the charge density work. He became the universal consultant, always available to explain the many relevant theoretical concepts to us experimentalists. As the meetings continued an increasing number of theoreticians became interested and joined, so much that the meetings now attract a fairly equal number of experimentalists and theoreticians.

Vedene Smith of Queens University
My contribution to the developments was rewarded in 1996 with the Aminoff Award of the Royal Swedish Academy of Sciences. At about that time the need for a comprehensive text to allow new students to enter the field became obvious. During a few years I spent much of my 'free' time to fill that gap, resulting in the book X-ray Charge Densities and Chemical Bonding, which was published by Oxford University Press in 1997 [21], and in a review article with Tibor Koritsanszky in Chemical Reviews [22]. In 2006 my co-workers completed the Charge Density Refinement program XD [23]. It replaced earlier less comprehensive codes and has been used in many applications. The field developed so strongly that I felt confident in 2009 to write a short report in Angewandte Chemie entitled 'Charge Densities Come of Age', concluding that 'Experimental studies of unusual bonds demonstrate how new light can be shed on longstanding issues in chemical bonding' [24].

Standing in line at the 2004 Gordon Conference at Mount Holyoke.
Among others: Kiyo Tanaka, Robert Stewart, Piero Macchi, Jochen Schneider, Richard Bader (hidden behind Jochen).
As the field matured, the revolutionary new tools which had become available, including lasers, fast computers, convenient low temperature equipment, synchrotron sources and others, offered the opportunity to return to my early love of the field of photocrystallography. Slow chemical reactions in crystals could be monitored by collecting a set of X-ray data after increasing times of laser exposure, but could one measure the atomic structure of molecular excited states with lifetimes of microseconds or less?
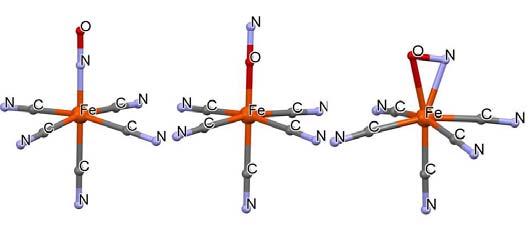
The nitroprusside anion and its photoinduced linkage isomers with an inverted nitrosyl group (center) and a side-bound one (right).
Sodium nitroprusside seemed an ideal case for a first study. It was believed to have a photoinduced excited state with a lifetime of more than two hours at low temperatures. We did indeed find a large structural change which we published as an excited state structure, but soon found out that we were actually dealing with a photo induced metastable linkage isomer in which the nitrosyl group was differently bonded to the iron atom, as illustrated in Fig. 8 [25,26]. The structure of sodium nitroprusside and the following excited state work was hailed by Chemical and Engineering News in its 100 years of X- ray crystallography issue of August 11, 2014 (http://cen.xraycrystals.org/) as one of their ten favorite X-ray structures which answered pressing chemical questions of their day. We continued with the studies of fleeting excited species with lifetimes of microseconds or less. To examine those we had to turn to synchrotron sources with their high brightness and 100 picosecond short pulses. Luck had it that I was a partner in the SUNY beamline at the National Light Source at Brookhaven National Laboratory. With a graduate student Chris Kim, a postdoc from Australia, Wilfred Fullager, a postdoc from France, Sebastien Pillet, and expert beamline scientist Guang Wu, we measured and solved the 50 μs 16K excited state structure of [Pt2(P2O5H2)4]4- and found a contraction of 0.28 Å in the Pt-Pt distance [27]. In 2000 we moved the time-resolved work to the third generation Advanced Photon Source (APS) at Argonne National Laboratory where Tim Graber constructed an excellent laser facility next to the diffractometer at beamline ID-15 of ChemMat CARS. The work is still continuing up to this day, now at the BioCars ID-14 beamline, leading to a series of excited state structures, including binuclear coordination complexes like the platinum complex [28,29], Cu(I)-metal-to-ligand- charge transfer complexes [30,31], and coinage metal compounds [32]. A great interaction was established with the Crystallographic laboratory headed by Prof Krzysztof Wozniak at the University of Warsaw, Poland, from which several highly capable well-trained graduates joined the work in Buffalo and at APS, several of which became first authors of some of our publications.
In addition to our continuing efforts in the Time-Resolved Diffraction field, which included the development of polychromatic Laue techniques at APS, following their application to macromolecular crystallography by Moffat and coworkers [33], I started a new research program involving polyoxotitanate nanoparticles in order to better understand the function of doped titanium oxide particles in photovoltaic cells. Initially with Jason Benedict [34], later, after he became an independent faculty member in our Department, with several most capable other co-workers. In particular Yang Chen was able to obtain a large variety of doped and undoped crystals by high-pressure solvothermal synthesis, the structure of which we use for theoretical calculations to explain their spectra and other properties [35,36,37]. The properties included photoinduced electron injection into the anode of a photovoltaic cell by electrochemical studies. This was a new technique for us, applied with expert help from Prof. David Watson and his group in our Department [38]. At a Rigaku meeting at Yale University in 2010 I met the local Solar Energy group, which led to a fruitful collaboration with joint publications, which is continuing at this time [39,40]. The effect of structurally-defined doping and chromophore attachment on spectral properties and interfacial charge transfer remain major issues to be addressed, and a continuing subject of our current studies.

With Pierre Becker at the Sagamore XVI meeting in Santa Fe, 2009, in which the field of structural dynamics was explicitly included.
Awards and Acknowledgments
I joined a series of highly-recognized X-ray crystallographers when I was awarded the triennial Ewald Prize of the International Union of Crystallography at the Florence IUCr Congress in 2006 for contributions to developing the charge density field and the crystallography of molecular excited states. Other awards were received in the USA, France, Japan and Poland. I want to acknowledge the continuous support of my wife Marguerite during the decades of the work, and the contributions of an international group of more than a hundred students and postdoctoral fellows, who have worked with me over the years and without whose participation the work would not have been possible. Many came from other continents. Several of those have returned home to start what have become major crystallographic centers. They include Yu Wang in the Chemistry Department at National Taiwan University, Claude Lecomte who established a major center of Advanced Crystallographic Research at the University Henri Poincaré in Nancy, Vaclav Petricek at the Institute of Physics of the Czech Academy in Prague, and T. N. Guru Row at the Indian Institute of Science in Bangalore. Long-term support by the National Science Foundation and the U.S. Department of Energy has been indispensable, as has been access to the many synchrotron and neutron facilities we have used over the years. My services as 1978 President of the American Crystallographic Association and President of the International Union of Crystallography from 1993-1996 have been most rewarding and have led to many new friendships.
References
.[1] Coppens, P 1997 Synch. Rad. News 10 26-30
.[2] Schmidt, G M J 1971 Pure Appl. Chem. 27 647-678
.[3] Hirshfeld, F L 1971 Acta Cryst. B27 769-781
.[4] Coppens, P and Hirshfeld, F L 1964 Isr. J. Chem. 2 117-119
.[5] Coppens, P, Leiserow.L and Rabinovi.D 1965 Acta Crystallogr. 181035-1038
.[6] Coppens, P 1967 Science 158 1577-1579
.[7] Coppens, P and Hamilton, W C 1968 Acta Cryst. 24 925-929
.[8] Coppens, P and Hamilton, W C 1970 Acta Cryst. A26 71-83
.[9] Stewart, R F 1970 J. Chem. Phys. 53 205-213
.[10] Stewart, R F, Davidson, E R and Simpson, W T 1965 J. Chem. Phys. 42 3175-3187
.[11] Stewart, R F 1973 J. Chem. Phys. 58 1668-1676
.[12] Hansen, N K and Coppens, P 1978 Acta Cryst. A34 909-921
.[13] Becker, P J and Coppens, P 1974 Acta Cryst. A30 129-147
.[14] Becker, P J and Coppens, P 1974 Acta Cryst. A30148
.[15] Becker, P J and Coppens, P 1975 Acta Cryst. A31417
.[16] Stevens, E D, Rys, J and Coppens, P 1977 Acta Cryst. A33333-338
.[17] Bader, R 1994 Atoms in Molecules. A Quantum Theory (New York, NY: Oxford University Press)
.[18] Gatti, C 2013 Phys. Scr. 87. DOI 10.1088/0031-8949/87/04/048102
.[19] Coppens, P.; Petricek, V.; Levendis, D.; Larsen, F. K.; Paturle, A.; Yan, G.; Legrand, A. D. 1987, Phys. Rev. Lett. 59 1695-1697
.[20] Gao, Y.; Lee, P.; Ye, J.; Bush, P.; Petricek, V.; Coppens, P. 1989 Physica C160, 431-438.
.[21] Coppens, P 1997 X-ray Charge Densities and Chemical Bonding ([Chester, England]; Oxford; New York: International Union of Crystallography ; Oxford University Press)
.[22] Koritsanszky, T S and Coppens, P 2001 Chem. Rev. 1011583-1628
.[23] Volkov, A, Macchi, P, Farrugia, L J, Gatti, C, Mallinson, P R, Richter, T and Koritsanszky, T 2006. XD2006 - A computer program package for multipole refinement,topological analysis of charge densities and evaluation of intermolecular energies from experimental and theoretical structure factors.
.[24] Coppens, P 2009 Angew. Chem. Int. Ed. 48 4280-4281
.[25] Carducci, M, Pressprich, M R and Coppens, P 1997 J. Am. Chem. Soc. 119 2669-2678
.[26] Coppens, P, Novozhilova, I and Kovalevsky, A 2002 Chem. Rev. 102861-884
.[27] Kim, C D, Pillet, S, Wu, G, Fullagar, W K and Coppens, P 2002 Acta Cryst.A58 133- 137
.[28] Coppens, P, Gerlits, O, Vorontsov, I I, Kovalevsky, A Y, Chen, Y-S, Graber, T, Gembicky, M and Novozhilova, I V 2004 Chem. Comm. 2144-2145
.[29] Makal, A, Trzop, E, Sokolow, J D, Kalinowski, J, Benedict, J B and Coppens, P 2011Acta Cryst. A67 319-326
.[30] Makal, A, Benedict, J, Trzop, E, Sokolow, J, Fournier, B, Chen, Y, Kalinowski, J A, Graber, T, Henning, R and Coppens, P 2012 J. Phys. Chem. A 116 3359-3365
.[31] Vorontsov, I I, Graber, T, Kovalevsky, A Y, Novozhiliva, I V, Gembicky, M, Chen, Y-S and Coppens, P 2009 J. Am. Chem. Soc. 131 6566-6573
.[32] Jarzembska, K, Kaminski, R, Fournier, B, Trzop, E, Sokolow, J, Chen, Y, Henning, R and Coppens, P 2014 Acta Crystallographica Section A 70 C774
.[33] Ren, Z, Bourgeois, D, Helliwell, J R, Moffat, K, Srajer, V and Stoddard, B L 1999 J. Synchrotron Rad. 6 891-917
.[34] Benedict, J B and Coppens, P 2010 J. Am. Chem. Soc. 1322938-2944
.[35] Chen, Y, Sokolow, J, Trzop, E, Chen, Y-S and Coppens, P 2013 J. Chin. Chem. Soc. 60 887-890
.[36] Chen, Y, Trzop, E, Makal, A, Sokolow, J D and Coppens, P 2013 Inorg. Chem. 52 4750-
4752
.[37] Coppens, P, Chen, Y and Trzop, E 2014 Chem. Rev. 1149645−9661
.[38] Jarzembska, K N, Chen, Y, Nasca, J N, Trzop, E, Watson, D F and Coppens, P 2014 Phys . Chem. Chem. Phys. 16 15792-15795
.[39] Snoeberger III, R C, Young, K J, Tang, J, Allen, L J, Crabtree, R H, Brudvig, G W, Coppens, P, Batista, V S and Benedict, J B 2012 J. Am. Chem. Soc. 1348911-8917
.[40] Negre, C F A, Young, K J, Oviedo, M B, Allen, L J, Sanchez, C G, Jarzembska, K N, Benedict, J B, Crabtree, R H, Coppens, P, Brudvig, G Wet al 2014 J. Am. Chem. Soc.136 16420-16429
|










